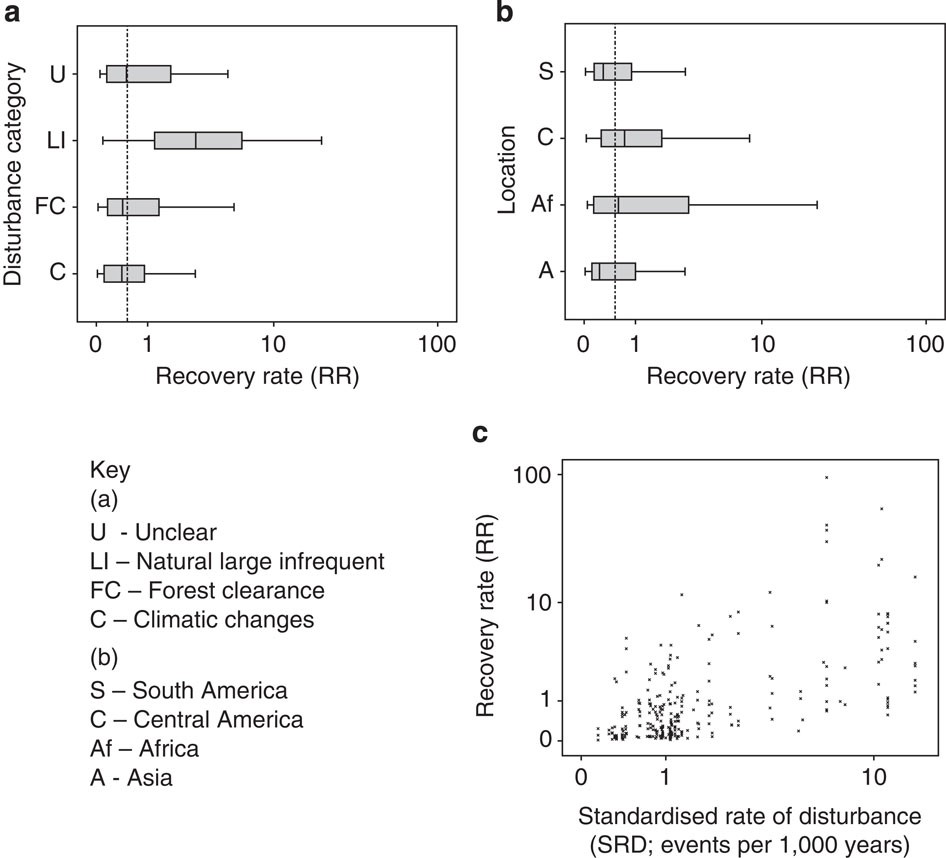
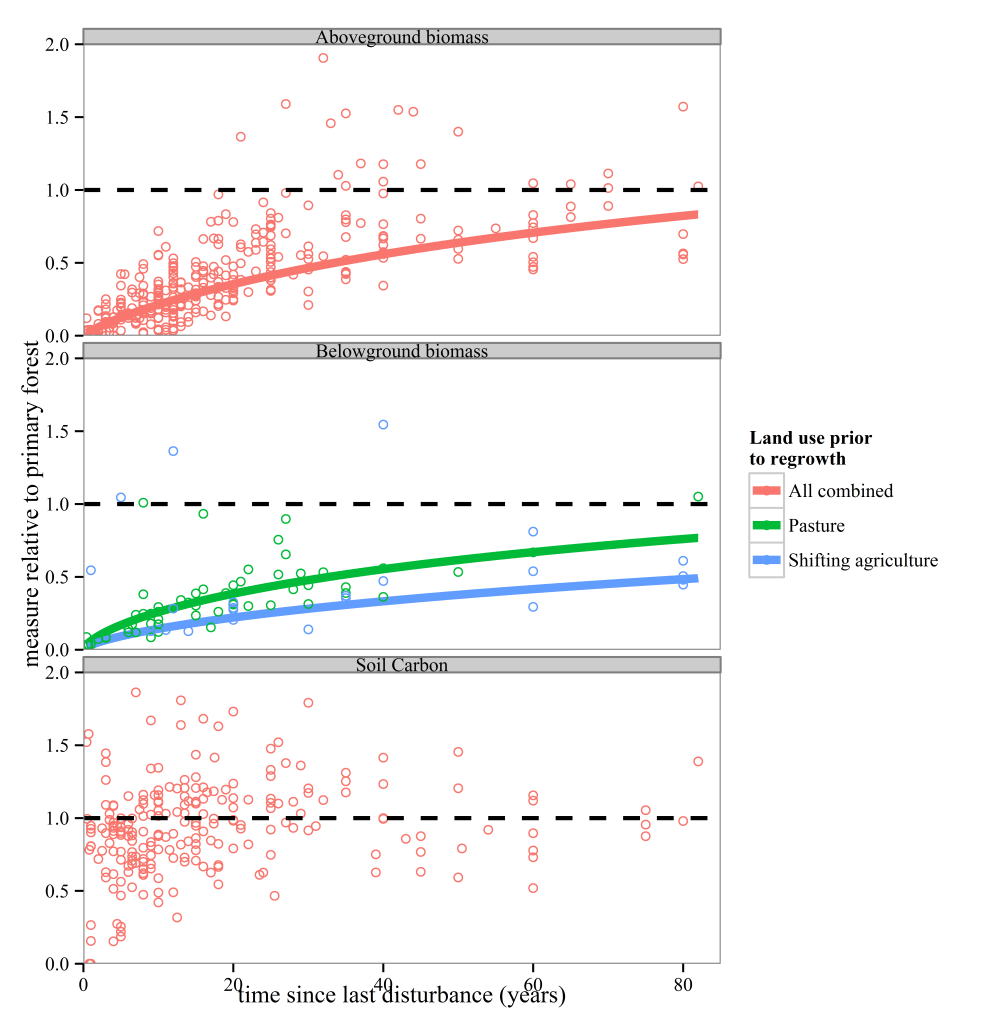
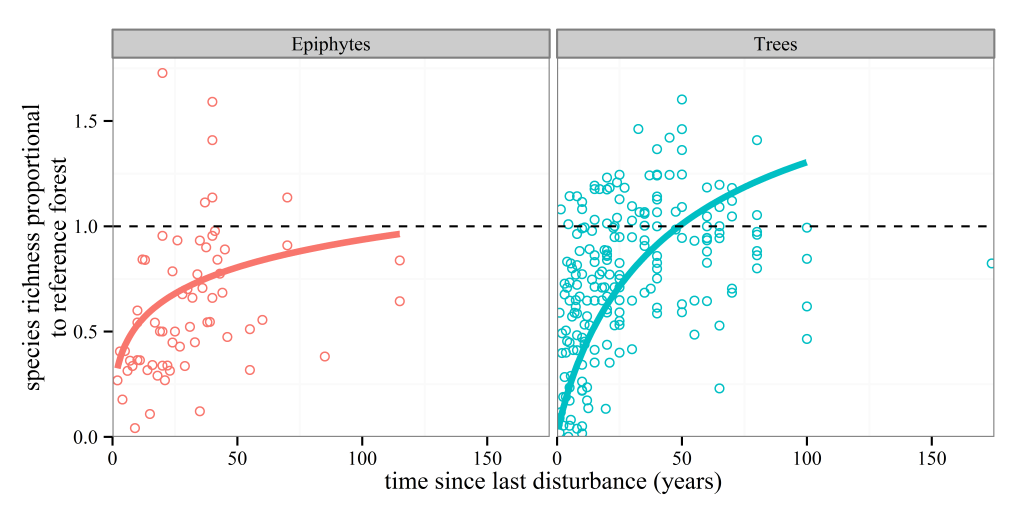
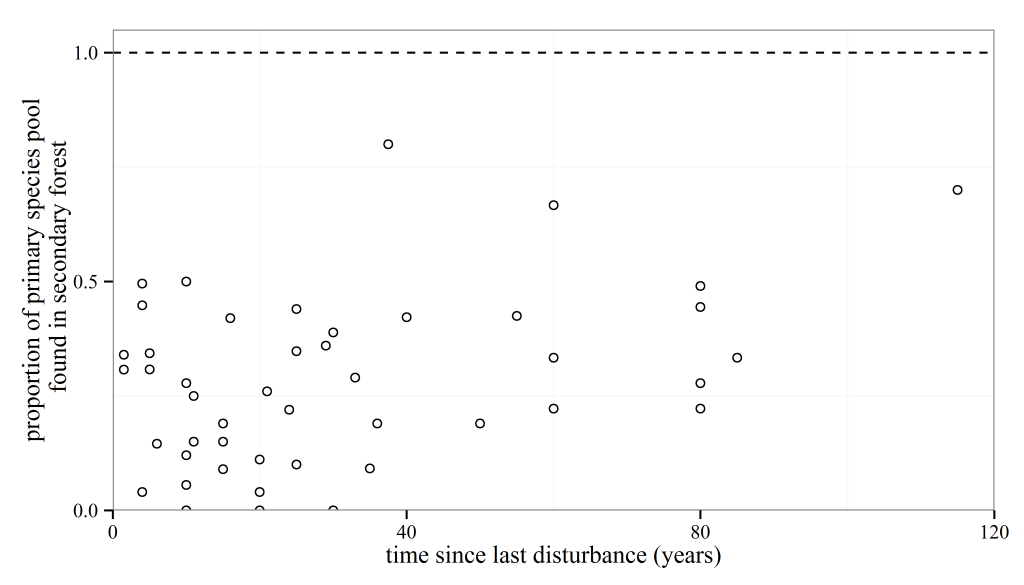
Anyone who knows anything about secondary forests will have come across the work of Robin Chazdon. She has inspired at least one forest ecologist, me, that forests recovering from major disturbances are a subject worthy of study. I’m sure she has done the same for many others out there. So, coming towards the end of my PhD I was excited to see a book that she had written summarising the topic was due to be released and using the last of my NERC funds I bought it. And then I moved house to Spain, where the book sat untouched and unloved in a box for the next year. After I came back to the UK last year, I found the book again and decided I should stop putting off reading it. I read it on trains, buses, on my sofa and occasionally in bed. I once fell asleep reading this book, though admittedly that was on the way back from the BES annual meeting in Edinburgh, and the gin from the previous night was probably the cause of my sleepiness rather than any bad writing.
The first thing to say is that this book is extremely comprehensive. Though it is not particularly lengthy, running to 316 pages of text, it covers a huge range of topics relating to forest regeneration from traditional knowledge and prehistoric forest transformations by humans to recovery pathways from fire, landslides, volcanic eruptions, logging, and agricultural use. There are also numerous sections on community assembly, functional traits, ecosystem function, and animal and plant interactions. The last section concentrates on reforestation and restoration of degraded forests, making a passionate plea for degraded forests to not be considered as wasteland.
For me the most fascinating parts of the book were those that covered traditional knowledge of forest regeneration and the history of human cultures in tropical forests – both subjects I knew practically nothing about before this book. I was captivated to read that the dayak people of Borneo have five words to define different stages of forest recovery – kurat uraq (1-3 year old scrub that forms after abandonment), kurat tuha (trees > 5 cm in diameter and 5-6 metres in height), kurat batang muda (trees 10-15 cm in diameter), kurat batang tuha (closed canopy secondary forest) and hutan bengkar (primary forest). As Chazdon points out this knowledge shows a striking resemblance to that of forest ecologists. Similarly, Mayan cultures in Central America and Soliga people in the Western Ghats have developed a subtle knowledge of the stages of forest succession. I have always been a bit skeptical of integrating traditional knowledge into ecological science, but this book convinced me that there could be some value to it.
Chazdon masterfully weaves together anthropology, archaeology and ecology in the discussion of prehistorical impacts of humans on tropical forests. She cites evidence of earthworks called geoglyphs similar to the Nazca lines found in the state of Acre in Brazil, swidden agriculture 20,000 years ago in Papua New Guinea and human populations in Central America to dispel the view that any forest is truly untouched. There are probably legacies of human use in most forests, we just can’t identify them. Based on this she, perhaps controversially, critiques recent work suggesting that mature tropical forest biomass density is increasing as a result of atmospheric carbon dioxide. Chazdon’s view is that this increase could well be as a result of recovery from unseen disturbances that happened generations ago.
The section on community turnover during succession is also excellent, with a detailed analysis of the characteristics of short- and long-lived pioneer and shade tolerant, late successional species. At points Chazdon playfully conjures up text resembling Shakespeare’s “All the world’s a stage” monologue: “The term successional stage is apt. Successional pathways can be viewed as an improvisational drama in several acts, with each act featuring a different set of actors. Some actors perform throughout the drama, but others have cameo appearances of only one act. Although each act sets the stage for the next, forest regeneration has no director and only a roughly sketched script creating a high degree of spontaneity, randomness and uncertainty.” These are amongst my favourite parts of the book, with metaphor mixing with a solid science to help things stick in your mind that might otherwise be easily forgotten.
If I have any criticism of the book, it is that it’s a bit repetitive. This is probably because Chazdon sees succession as ‘an improvisational drama in several acts’ and so the book relies on case studies, rather than synthesising current knowledge to form generalities. However, I think that the repetition helps if you just want to dip in and out of chapters – I don’t think it is necessarily written to be read cover to cover like I did.
That aside if you are interested in the dynamics of forests in any way this book is essential reading. There is no better summary of current thinking on tropical forest succession out there.