Roads are generally terrible for biodiversity. They fragment habitat, can increase habitat loss and hunting as a result of increased access, and cause direct loss of biodiversity as a result of collisions. However, not all roads are the same. Some are massive, permanent structures, while others are temporary, dirt tracks that may seemingly disappear once they fall into disuse.
One example of ephemeral roads is those that logging companies construct in tropical forests to provide access and transport of logs. There has long been concern that these roads can increase the risk of fires occurring, as well as increasing access for hunting, and other forms of forest exploitation. However, in a recent(ish) paper* has shown that some of the negative effects of logging roads are relatively transient.
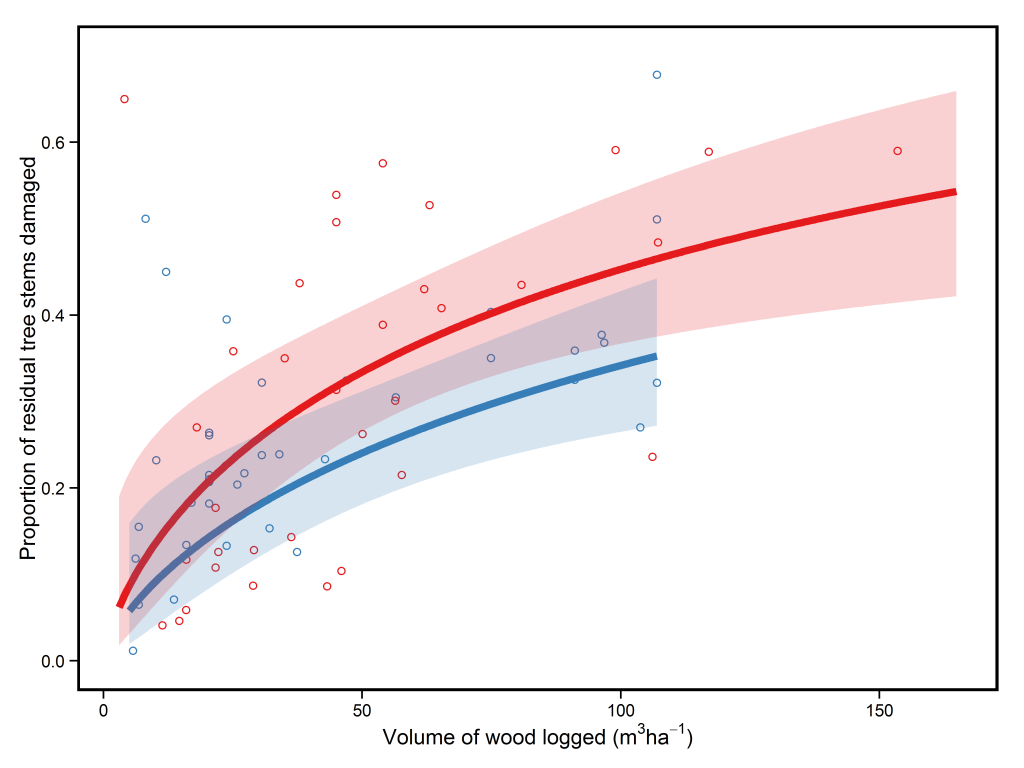
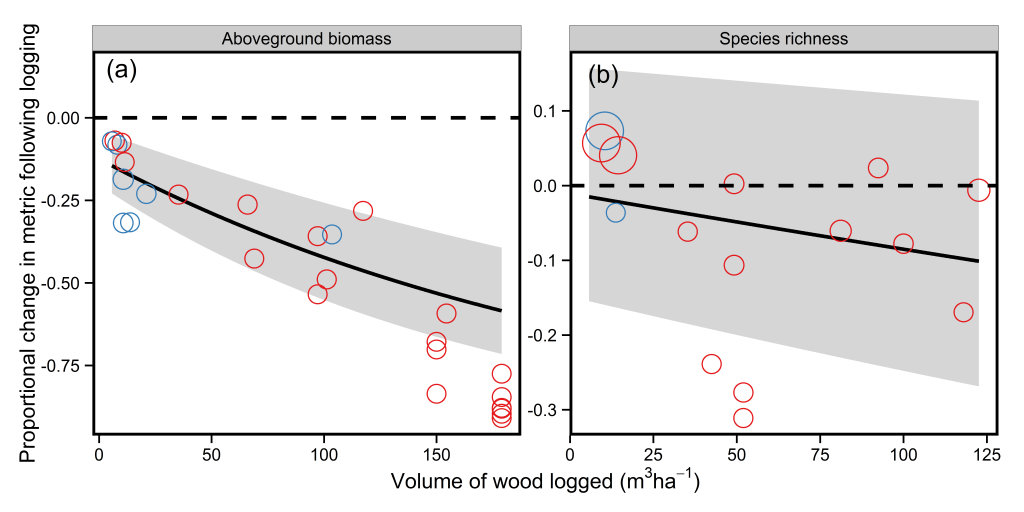
In the paper, Fritz Kleinschroth and colleagues showed that in Central African forests, after 30 years of recovery logging roads had similar canopy cover, species diversity, and leaf litter to logged forests nearby* . However, the amount of carbon stored in the form of biomass lagged behind and was only 6% of that seen for logged forests after 30 years of recovery. At this rate, biomass recovery would take more than 300 years.This incredibly slow recovery at first appears puzzling, given that secondary forests, which have had almost all their trees cut down in the past and turned into agricultural fields, tend to take between 60-100 years to recover biomass to pre-disturbance levels (see here for a blog post and here for a recent paper on this). However, the probable cause of this delayed recovery is the compaction of soils on the roads by heavy vehicles which reduces seed germination and root growth***. Taken together the authors suggest these results indicate that logging roads have the potential to act as areas in which timber species could regenerate and that they may become inaccessible to hunters within 10 years.
So how does this study compare to similar ones carried out previously? Firstly, this study is a little different because it is one of the few that used chronosequences to assess recovery, and so the only study I know of which can assess longer-term dynamics on logging roads. However, other studies present a similar picture for the recovery of biomass and forest structure – forest canopy cover recovers relatively quickly (see here and here), but biomass and basal area lag behind (see here and here). The major difference between this study and previous ones is that it presents a more optimistic outlook of biodiversity. Previous studies have estimated that species richness may be 50-95% lower on abandoned logging roads when compared to logged forests (see here, here, and here). As such, the relatively fast recovery of species richness shown by Kleinschroth and colleagues appears to be outside of the norm, and further similar studies will be needed to see whether the pattern of recovery shown in this paper is an outlier. So we can’t really give a solid answer to the question posed in the title of this post – sorry about that.
While results vary from study to study it is obvious that more efforts need to be made to reduce the number of logging roads, their initial impacts to forests, and to help them recover once they are abandoned. In order to reduce the number of roads, reduced-impact logging could be used. This method, which I have been accused of disliking in the past, seems to be very successful in reducing the number of roads in logging concessions where it has been used (see here for an example of this). This is done by producing a plan for road construction prior to any trees being cut, rather than the ad-hoc approach often taken in conventional logging. Reducing the impact of roads could be done by limiting their width. Finally, improving recovery could be done by planting seedlings/saplings on former logging roads, as well as reducing access to roads.
One suggestion that the authors made in their paper that I really like is to re-use logging roads when forests are re-logged. Given that logging typically occurs every 30 years, this would allow some time for recovery of biodiversity on the roads but clearing them would reduce the damage caused by their construction spreading to other areas of the forest.
*I admit it, I’ve been terrible at keeping up with my reading recently.
**John Healy and Fritz have written a nice summary of their paper on the website “The Conversation” which is well worth a read.
***Anecdotal, I know, but I have seen similar things on restored salt marshes in the UK where diggers have been used to breach sea walls. At least for the ones I remember, this resulted in reduced vegetation cover.