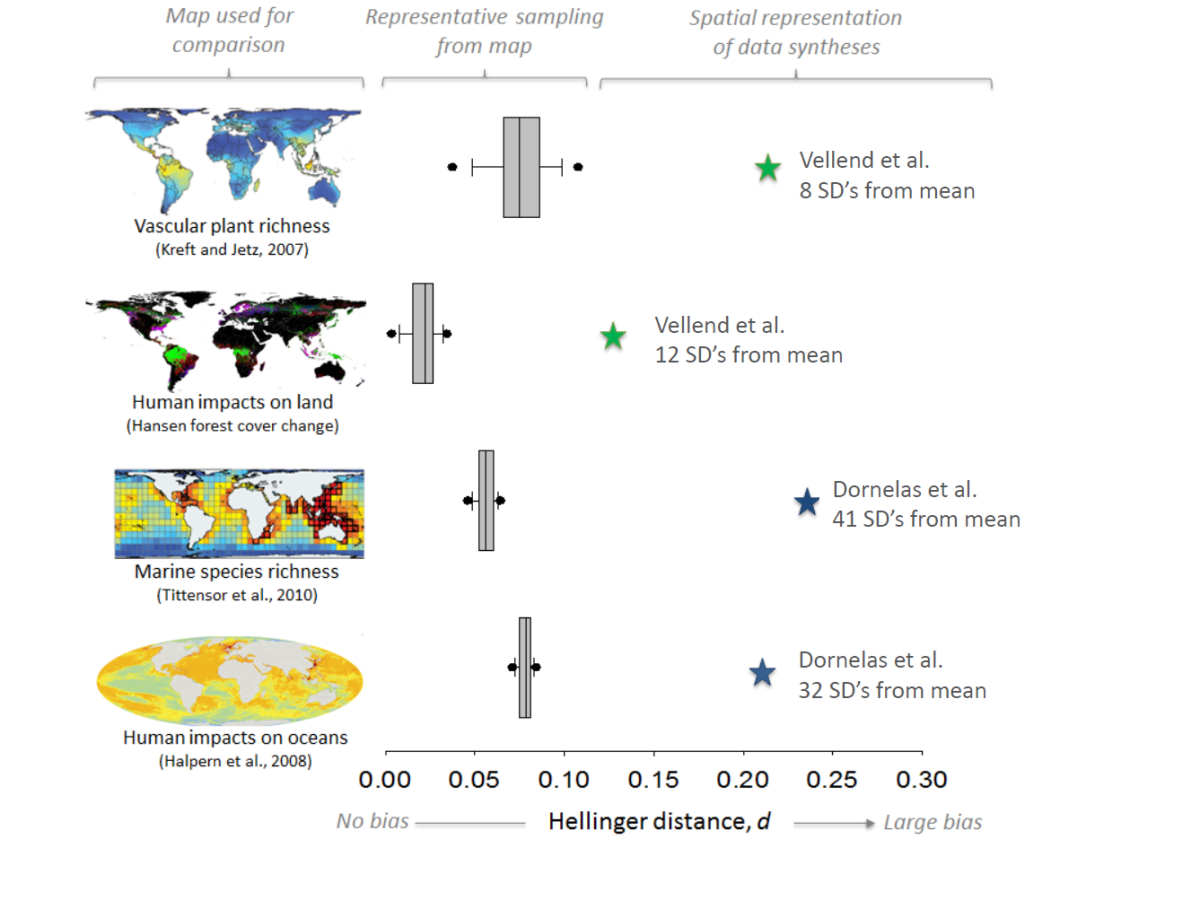
Almost all ecological research of human impacts on biodiversity looks at changes after they have happened. To do this, researchers usually compare a site where some kind of disturbance has happened to a nearby undisturbed site. This method is called space-for-time substitution. The assumption of this approach is that the only thing that differs between sites is this disturbance. However, this assumption is often incorrect. Sites may have had very different biodiversity before any disturbances, which can lead to under- or over-estimations of biodiversity changes as a result of human impacts. One result of this is that we aren’t really sure how tropical logging alters the composition of ecological communities. These problems are likely to be particularly acute when habitat fragmentation limits dispersal to some sites.
Up until recently there had been little work comparing how the results from space-for-time methods compare to methods that compare sites before and after disturbances. However, last week an elegantly designed study was published in the Journal of Applied Ecology which aimed to examine just this in the context of logging in Brazil. The paper aimed to compare space-for-time methods to before-after-control-impact (BACI) methods. Critically BACI studies measure biodiversity at sites at least once before the disturbance of interest takes place. Researchers then return to sites and remeasure them after the disturbance. Importantly both sites impacted by the disturbance and control sites are surveyed on both occasions. Using this method allows researchers to disentangle the effects of disturbances and any differences between sites prior to disturbance – a key advantage over space-for-time methods.
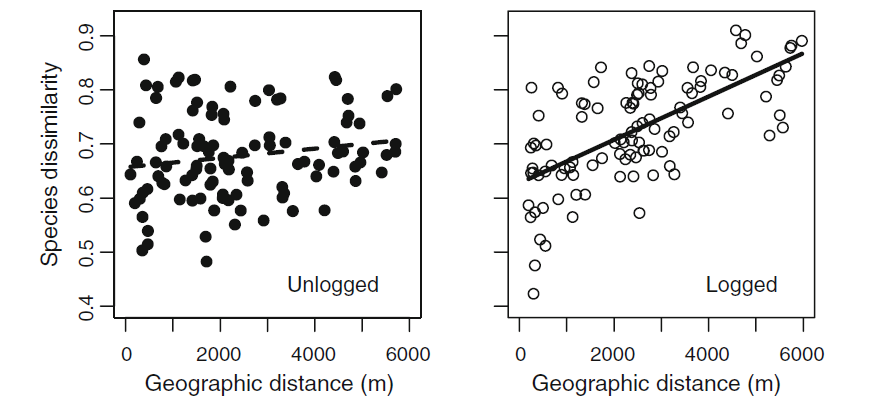
The paper by Filipe França and colleagues examined the differences in results obtained for space-for-time and BACI methods when looking at changes in dung beetle biodiversity in tropical logged forests in Brazil. To do this they surveyed 34 locations in a logging concession, 29 of which were subsequently logged at a variety of intensities. The intensity of logging (the number of trees/volume of wood removed per hectare) is a very important determinant of the impact of logging on biodiversity and carbon (see previous posts on this here and here). They then went back and re-surveyed these locations one year later. From the data collected, they calculated changes in species richness, community composition and total biomass of dung beetles.

When comparing space-for-time and BACI the paper found that BACI characterised changes in biodiversity significantly better than space-for-time methods. Critically, space-for-time methods underestimated the relationship between logging intensity and biodiversity losses, with changes in species richness twice as severe as estimated by space-for-time (see Figure 1). BACI methods also consistently provided higher explanatory power and steeper slopes between logging intensity and biodiversity loss.
So what does this mean for how we do applied ecology? I think it is clear that we need to employ BACI methods more often in the future. However, BACI comes with logistical and financial constraints. Firstly, it is virtually impossible to predict where disturbances are going to happen before they occur. As a result, Franca and colleagues think that if we want to carry out more BACI research in the future, we need to develop closer ties with practitioners. This will involve building relationships with logging and oil palm companies, as well as agricultural businesses and property developers. This may make some researchers uncomfortable, but we need to do this if we are to provide robust evidence for decision makers. Secondly, BACI studies take longer to carry out, so we need to convince those that hold the purse strings that they are worth investing in.
BACI is clearly something we should be using more often but does this mean that space-for-time approach is useless? Should we even be using space-for-time methods at all? I’m not being hyperbolic just to get some attention- some have argued that we should stop using chronosequences altogether because ecological succession is unpredictable. After momentarily going into a bit a crisis about this when I read some papers on succession last year, I have come to a slightly different conclusion. Space-for-time substitution sometimes predicts temporal changes well, but sometimes it doesn’t. What we need is to work out when the use of space-for-time approaches are acceptable, and when it would be better to use temporal methods. Reviews have highlighted that as ecosystems increase in complexity space-for-time methods become less useful for monitoring changes in biodiversity. For example, large local species pools mean that post-disturbance colonisation may be very variable between sites. This problem is compounded in fragmented landscapes where there are barriers to dispersal of seeds and animals. Every additional layer of complexity makes post-disturbance dynamics more and more difficult to predict. Ultimately, the best way to address this problem is through some kind of synthesis.
Working out when space-for-time approaches are useful and when they are not is not something we are going solve overnight. Before we can review the evidence, we need some evidence in the first place. This is part of the reason why papers like the one by França and colleagues that I’ve discussed here are vitally important. So next time you think about designing a study see if you can assess how the results from temporal methods compare those from space-for-time methods. The results might just take you by surprise.
Filipe França & Hannah Griffiths have written a great post on the Journal of Applied Ecology blog going into more detail about the implications of their study. I strongly recommend you give it a look.